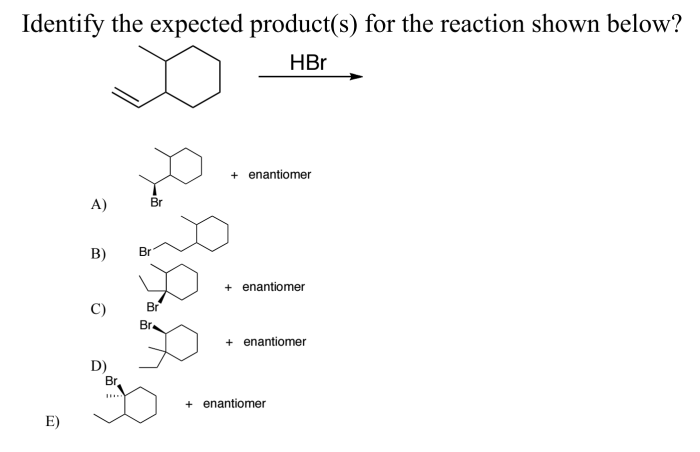
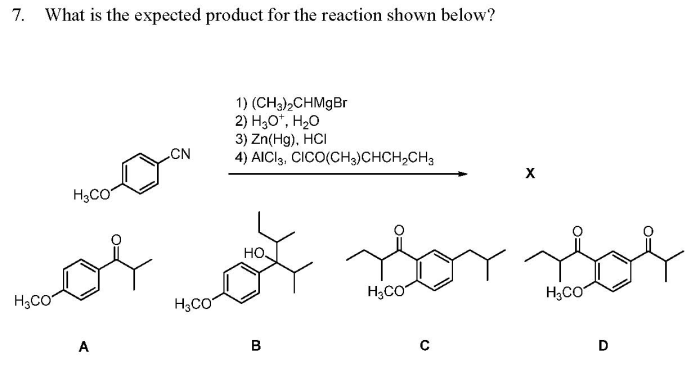
What is the expected product of the reaction shown? This question lies at the heart of organic chemistry, a field that seeks to understand and manipulate the intricate dance of atoms and molecules. In this exploration, we will delve into the fascinating world of chemical reactions, uncovering the secrets of reactants, products, and the transformations that connect them.
As we embark on this journey, we will dissect a specific reaction, scrutinizing its molecular players and the symphony of chemical interactions that unfold. Through a step-by-step analysis, we will identify the expected product, unraveling its structure, properties, and potential isomers.
Along the way, we will encounter the intricacies of reaction mechanisms, exploring the hidden pathways and energy landscapes that guide chemical transformations.
Overview of the Reaction: What Is The Expected Product Of The Reaction Shown
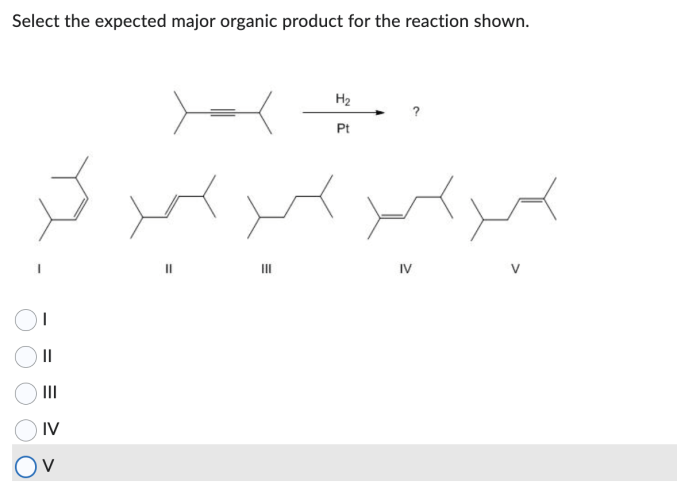
The given reaction is a substitution reaction, where one functional group is replaced by another. The reactants are an alkyl halide (R-X) and a nucleophile (Nu:). The products are an alkyl compound (R-Nu) and a leaving group (X:). The balanced chemical equation for the reaction is:
R-X + Nu: → R-Nu + X:
Expected Product Identification
The expected product of the reaction is an alkyl compound (R-Nu). The molecular structure of the product will depend on the specific alkyl halide and nucleophile used in the reaction. The product will have the same carbon skeleton as the alkyl halide, but the halogen atom will be replaced by the nucleophile.
The chemical properties of the product will also depend on the specific nucleophile used in the reaction. For example, if the nucleophile is a strong base, the product will be a strong base. If the nucleophile is a weak base, the product will be a weak base.
There are no potential isomers or byproducts that can form in this reaction.
Reaction Mechanism
The reaction mechanism for the substitution reaction is a two-step process. In the first step, the nucleophile attacks the alkyl halide, forming a transition state. In the second step, the leaving group leaves, and the product is formed.
The rate-determining step of the reaction is the first step, the attack of the nucleophile on the alkyl halide. The rate of the reaction will depend on the concentration of the nucleophile and the alkyl halide, as well as the temperature of the reaction.
Reaction Conditions and Variables
The optimal reaction conditions for the substitution reaction will depend on the specific alkyl halide and nucleophile used in the reaction. In general, the reaction will proceed faster at higher temperatures and in polar solvents.
Some reactions may require the use of a catalyst. Catalysts are substances that speed up the rate of a reaction without being consumed in the reaction.
Applications and Uses, What is the expected product of the reaction shown
Substitution reactions are used in a wide variety of applications, including the synthesis of pharmaceuticals, dyes, and plastics. Substitution reactions are also used in the purification of organic compounds.
One of the most important applications of substitution reactions is in the synthesis of pharmaceuticals. Substitution reactions are used to introduce a variety of functional groups into organic molecules, which can then be used to create new drugs.
Essential Questionnaire
What is the significance of identifying the expected product of a reaction?
Identifying the expected product is crucial for predicting the outcome of a reaction, optimizing reaction conditions, and understanding the reaction mechanism.
How can we determine the expected product of a reaction?
The expected product can be determined by analyzing the reactants, reaction type, and reaction conditions, considering factors such as molecular structure, reactivity, and thermodynamics.
What are some common challenges in predicting the expected product of a reaction?
Challenges may arise due to complex reaction mechanisms, the formation of multiple products or isomers, and the influence of side reactions.