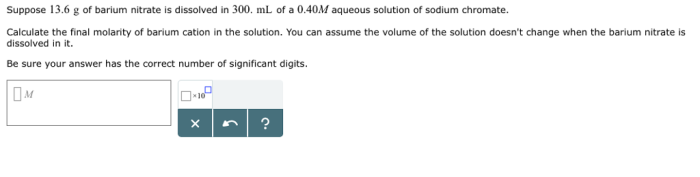
Barium nitrate and sodium chromate, two indispensable chemical compounds, occupy a prominent position in the realm of science and industry. This comprehensive exploration delves into their fundamental properties, reactivity, diverse applications, and essential safety considerations, providing a thorough understanding of these remarkable substances.
These chemicals exhibit distinct characteristics, with barium nitrate existing as a colorless solid at room temperature and sodium chromate appearing as a yellow crystalline powder. Their chemical formulas, Ba(NO3)2 and Na2CrO4 respectively, hint at their molecular compositions and pave the way for further investigation into their chemical behavior.
Chemical Properties: Barium Nitrate And Sodium Chromate
Barium nitrate and sodium chromate are inorganic compounds with distinct chemical properties:
Chemical Formulas
- Barium nitrate: Ba(NO3)2
- Sodium chromate: Na2CrO4
Physical States
- Barium nitrate: Colorless solid at room temperature
- Sodium chromate: Yellow solid at room temperature
Molar Masses
- Barium nitrate: 261.32 g/mol
- Sodium chromate: 161.98 g/mol
Solubility in Water
- Barium nitrate: Highly soluble
- Sodium chromate: Moderately soluble
Reactivity

Barium nitrate and sodium chromate exhibit varying degrees of reactivity:
Reactivity of Barium Nitrate
- Reacts with sulfuric acid to form barium sulfate and nitric acid
- Decomposes upon heating to produce barium oxide and nitrogen dioxide
Reactivity of Sodium Chromate
- Reacts with lead nitrate to form lead chromate (a yellow precipitate)
- Reacts with silver nitrate to form silver chromate (a red precipitate)
Stability and Decomposition
- Barium nitrate is stable at room temperature but decomposes when heated
- Sodium chromate is relatively stable but can decompose under certain conditions
Behavior When Heated
- Barium nitrate decomposes into barium oxide and nitrogen dioxide when heated
- Sodium chromate undergoes dehydration when heated, losing water molecules
Applications
Barium nitrate and sodium chromate have diverse industrial applications:
Applications of Barium Nitrate
- Pyrotechnics: Used to produce green flames
- Glass manufacturing: Acts as a refining agent
Applications of Sodium Chromate
- Pigments: Used to produce yellow pigments for paints and ceramics
- Metal treatments: Used as an anti-corrosive agent
- Wood preservatives: Used to protect wood from rot and decay
Analytical Chemistry
- Barium nitrate: Used to detect sulfate ions
- Sodium chromate: Used as an indicator in redox titrations
Safety Considerations
Barium nitrate and sodium chromate pose potential hazards:
Hazards
- Barium nitrate: Toxic if ingested, can cause respiratory irritation
- Sodium chromate: Carcinogenic, toxic if ingested
Safety Precautions
- Handle with gloves and protective clothing
- Store in a well-ventilated area
- Dispose of properly according to local regulations
Environmental Impact, Barium nitrate and sodium chromate
- Barium nitrate: Can contaminate soil and water sources
- Sodium chromate: Toxic to aquatic organisms
Regulations
- Both chemicals are regulated by various agencies worldwide
- Follow all applicable regulations for handling, storage, and disposal
FAQ Explained
What are the molar masses of barium nitrate and sodium chromate?
Barium nitrate: 261.32 g/mol, Sodium chromate: 161.98 g/mol
Are barium nitrate and sodium chromate soluble in water?
Yes, both barium nitrate and sodium chromate are soluble in water.
What is a common industrial application of barium nitrate?
Barium nitrate is widely used in pyrotechnics due to its ability to produce green-colored flames.
What safety precautions should be taken when handling sodium chromate?
Sodium chromate is toxic and should be handled with care, avoiding contact with skin and eyes.