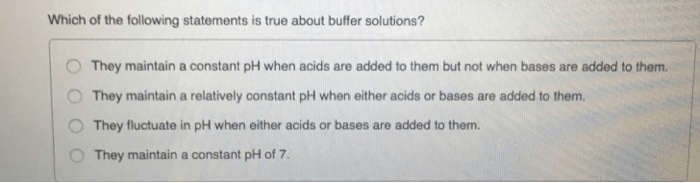
Which of the following statements about buffer solutions is true? Buffer solutions play a crucial role in maintaining pH stability and find diverse applications across various fields. In this comprehensive guide, we delve into the characteristics, preparation, and limitations of buffer solutions, providing a thorough understanding of their significance and practical implications.
Buffer solutions are defined by their ability to resist changes in pH upon the addition of small amounts of acid or base. They consist of a weak acid and its conjugate base or a weak base and its conjugate acid, and their pH range typically falls between 3 and 11. The effectiveness of a buffer solution depends on its buffering capacity, which is determined by the concentration of the weak acid and its conjugate base or the weak base and its conjugate acid.
Understanding Buffer Solutions

Buffer solutions are aqueous solutions that resist changes in pH when small amounts of acid or base are added. They play a crucial role in maintaining pH stability in various chemical and biological systems.
Common buffer solutions include:
- Acetate buffer (pH 4-6)
- Phosphate buffer (pH 7-9)
- Carbonate buffer (pH 9-11)
These buffers find applications in:
- Calibrating pH meters
- Controlling pH in biochemical reactions
- Preserving biological samples
Characteristics of Buffer Solutions
Buffer solutions exhibit specific characteristics that define their effectiveness:
- pH Range:The range of pH values over which a buffer can effectively resist pH changes.
- Buffering Capacity:The ability of a buffer to neutralize added acid or base without significant pH change. It depends on the concentration of the buffer components.
- pKa:The negative logarithm of the acid dissociation constant of the weak acid or base used to prepare the buffer. It determines the pH range of the buffer.
Factors influencing buffer effectiveness include:
- Buffer concentration
- Temperature
- Ionic strength
Preparation of Buffer Solutions
Buffer solutions can be prepared using various methods:
- Titration:Adding a strong acid or base to a weak acid or base until the desired pH is reached.
- pH Meter:Using a pH meter to monitor the pH and adding acid or base as needed to achieve the target pH.
Accurate measurements and calibration of pH meters are crucial for preparing buffers with the desired pH.
Applications of Buffer Solutions: Which Of The Following Statements About Buffer Solutions Is True

Buffer solutions have diverse applications in various fields:
Chemistry
- Calibrating pH meters
- Controlling pH in chemical reactions
- Separating and purifying proteins
Biology
- Maintaining pH in cell culture
- Buffering enzymes in biochemical assays
- Preserving biological samples
Medicine, Which of the following statements about buffer solutions is true
- Regulating pH in blood and other body fluids
- Formulating drug solutions
- Maintaining pH in medical devices
Limitations of Buffer Solutions

Buffer solutions have certain limitations:
- Limited pH Range:Buffers can only resist pH changes within their specific pH range.
- Temperature Sensitivity:The pH of buffers can change with temperature.
- Dilution Effects:Diluting a buffer can reduce its buffering capacity.
Using buffers outside their optimal conditions can result in inaccurate pH measurements or compromised performance in sensitive applications.
Frequently Asked Questions
What is the primary function of a buffer solution?
To resist changes in pH upon the addition of small amounts of acid or base, maintaining pH stability.
What factors influence the effectiveness of a buffer solution?
The concentration of the weak acid and its conjugate base or the weak base and its conjugate acid.
What are some common applications of buffer solutions?
Calibrating pH meters, controlling pH in chemical reactions, and maintaining pH levels in biological systems.